Toxicogenomics Analysis Based on DCL™
Background & Overview

For the pharmaceutical industry, toxicity (safety) is one of the important reasons for stopping drug development. Preclinical toxicology studies study the relationship between drug exposure and toxicity in animal models. However, this does not explain the mechanism of toxicity of the compound, making it impossible for toxicologists to predict the toxicity of new compounds in humans.
Toxicogenomics based on stable isotope labeling is an economical, rapid, safe, and effective toxicity detection method that can explain the toxicity mechanism of compounds. The method combines traditional toxicology with other disciplines such as genetics, transcriptomics, proteomics, metabolomics, and bioinformatics, using the equivalence of stable isotope labeling to elucidate the potential impact of chemical modes of action and gene-environment interactions.
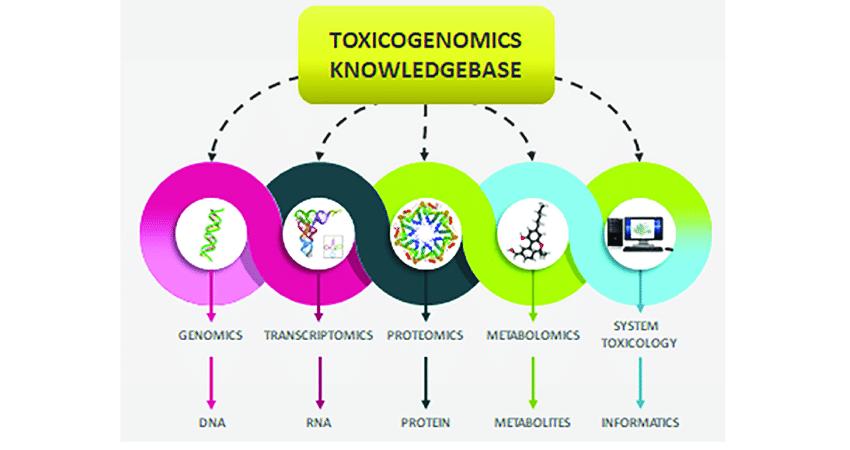 Fig.1 Schematic diagram showing flow of toxicogenomics knowledgebase.[1]
Fig.1 Schematic diagram showing flow of toxicogenomics knowledgebase.[1]
BOC Sciences researchers were able to use deuterium-labeled compounds to find key genes responsible for toxic effects and efficiently analyze the relationship between marker genes and target organ toxicity.
Our Services
Our toxicogenomics combined with deuterated compounds has played a breakthrough role in specific toxicity, experimental pathology, risk assessment of mixtures, immunotoxicology, neurotoxicology, and predictive toxicology. Our capabilities include the following:
Specific toxicity studies
Uncover the chemically specific ways of changing gene expression, that is, different chemicals are expressed by different genes, thereby facilitating toxicological classification and mechanism research.
Experimental pathology
Our focus on large-scale differential gene expression (DGE) data that can help pathologists apply DGE to drug discovery may better reveal potential desirable (therapeutic) and undesirable (toxic) responses.
Risk assessment of mixtures
Uncertainty is reduced by providing combined toxicity biomathematical model multicomponent data instead of pairwise interaction markers during mixed hazard assessment.
Immunotoxicology
Solve the problems that need to be solved in current immunotoxicology research, including understanding the immune system itself (including control system network and relationship with other systems); integration and classification of assay strategies; identification of substances that affect the immune system at a certain dose level in some cases, etc.
Neurotoxicology
Utilize a tiered testing system to complete three tiers of testing including chemical screening, specific neurotoxicity testing, and determination of the level of no visible adverse effects. Toxicogenomics technology facilitates neurotoxicity research.
Predictive toxicology
Predict the possible toxic effects of chemicals. Discovering the effects of toxicants in the reversible stage of initial exposure of toxicants or predicting the possible toxic effects of chemicals before they are used provides a scientific basis for taking corresponding preventive measures to avoid hazards caused by toxicants.
Technical Advantages
- Accurately evaluate the potential toxicity of drug candidates
- High-throughput, providing rich and comprehensive toxicological information on the effects of compounds on multiple targeted organs
- Throughout all stages of drug development, including compound screening, toxicological mechanism of action studies, toxicity target or biomarker discovery, and potential toxicity prediction
- Systematic elucidation of toxic effects with reliable and reproducible results
If you are interested in our services, please contact us immediately, then fill in the complete inquiry form, and we will reply to you as soon as possible.
Reference
- Yahya F A, et al. A brief overview to systems biology in toxicology: The journey from in to vivo, in-vitro and–omics. Journal of King Saud University-Science. 2021, 33(1): 101254.


 Fig.1 Schematic diagram showing flow of toxicogenomics knowledgebase.[1]
Fig.1 Schematic diagram showing flow of toxicogenomics knowledgebase.[1]
