Deuteration of Alcohols Based on DCL™
Background & Overview
Deuterium-labeled compounds have important research significance in the fields of organic synthetic chemistry, medicinal chemistry, and life sciences. In order to overcome the defects of poor metabolic stability and high toxicity of traditional pharmaceutical compounds, medicinal chemists from various countries have turned their hope to deuterated compounds. Selective deuterium substitutions play a role in altering the metabolism and absorption properties of drugs due to kinetic isotope effects. Alcohol compounds and their derivatives are important fragments for the synthesis of many active compounds. Therefore, we have reason to believe that the synthesis of deuterated alcohols is of great significance for deuterated drugs with improved safety and/or efficacy.
Application of Deuterated Alcohols
The synthesis of deuterium-labeled alcohols is an important transition in organic synthesis, as deuterated drugs and biologically active organic molecules play crucial roles in the metabolism of alcohols and enzymes. In addition, deuterium-labeled alcohols can be used as NMR solvents and reliable chemical probes for analyzing complex mixtures. For example, β-deuterated alcohol can be used as an internal standard for the analysis of olive leaf extracts.
Existing Strategy for the Synthesis of Deuterated Alcohols
Conventional selective deuterated alcohols are synthesized from aldehyde or carbonyl derivatives of alcohols using reducing reagents such as NaBD4, LiAlD4, and SiDMe2Ph/F through an extended multi-step procedure, which results in huge hazardous waste, and extremely high cost. On top of that, the scope of applicability of the reaction is also more limited. Therefore, a method for the direct synthesis of selective deuterated alcohols based on H/D exchange reaction and deuterium oxide was developed. For example, a combination of 5% ruthenium/carbon (Ru/C), hydrogen, and deuterium oxide (D2O) can achieve deuteration of the hydroxyl group on the vicinal carbon.
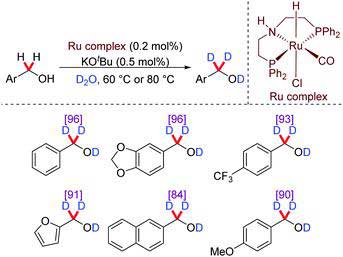 Fig.1 Ru-Catalysed deuteration of aryl methanols.[3]
Fig.1 Ru-Catalysed deuteration of aryl methanols.[3]
DCL™ in Deuteration of Alcohols
Our DCL™ technology platform focuses on deuterium labeling of various types of alcohol compounds for you. All synthetic routes are constructed based on simple and efficient design ideas, which can greatly improve the delivery efficiency of customized products to advance your project progress. Whether it is aryl alcohols, heteroaryl alcohols, linear fatty alcohols, monohydric, dihydric, polyhydric alcohols, or a variety of primary, secondary and tertiary alcohols, our advanced technology platform enables high selectivity deuteration.
References
- Jiménez T, et al. Radical reduction of epoxides using a titanocene (III)/water system: synthesis of β‐deuterated alcohols and their use as internal standards in food analysis. 2010.
- Chatterjee B, et al. Ruthenium catalyzed selective α-and α, β-deuteration of alcohols using D2O. Organic letters. 2015, 17(19): 4794-4797.
- Prakash G, et al. C–H deuteration of organic compounds and potential drug candidate. Chemical Society Reviews. 2022.
Customer Intellectual Property Protection
BOC Sciences has always regarded intellectual property as the most valuable asset of the company and its customers. We have signed non-disclosure agreements with customers and employees before the project starts, and provide synthetic route design and synthesis services in strict accordance with the terms of the non-disclosure agreement, striving to provide customers with target compounds in the shortest time possible.


 Fig.1 Ru-Catalysed deuteration of aryl methanols.[3]
Fig.1 Ru-Catalysed deuteration of aryl methanols.[3]

