Deuteration of Aldehydes Based on DCL™
Background & Overview
Deuterium labeling technology has become a long-standing and important research tool in numerous disciplines, especially in medicinal chemistry. In fact, the incorporation of deuterium can often alter the absorption, distribution, metabolism, excretion and toxicity properties of target compounds. Therefore, the design and use of deuterated analogs to modify chemical or biological drugs while increasing their overall potency holds great potential for the discovery of better treatments.
Aldehydes are ubiquitous in pharmaceutical and organic synthesis, and aldehyde groups can be transformed into a variety of other building blocks through specific chemical transformations. Deuterated aldehydes serve as ideal deuterated synthetic building blocks for building more complex molecular structures. Therefore, it is of great significance to develop efficient synthetic methods to obtain deuterated aldehydes.
Existing Strategy for the Synthesis of Deuterated Aldehydes
Classical methods
Traditional methods are mainly based on selective reduction of carboxylic acid derivatives using appropriate reducing agents or cascade reactions involving LiAlD4 reduction and selective oxidation to obtain deuterated aldehydes.
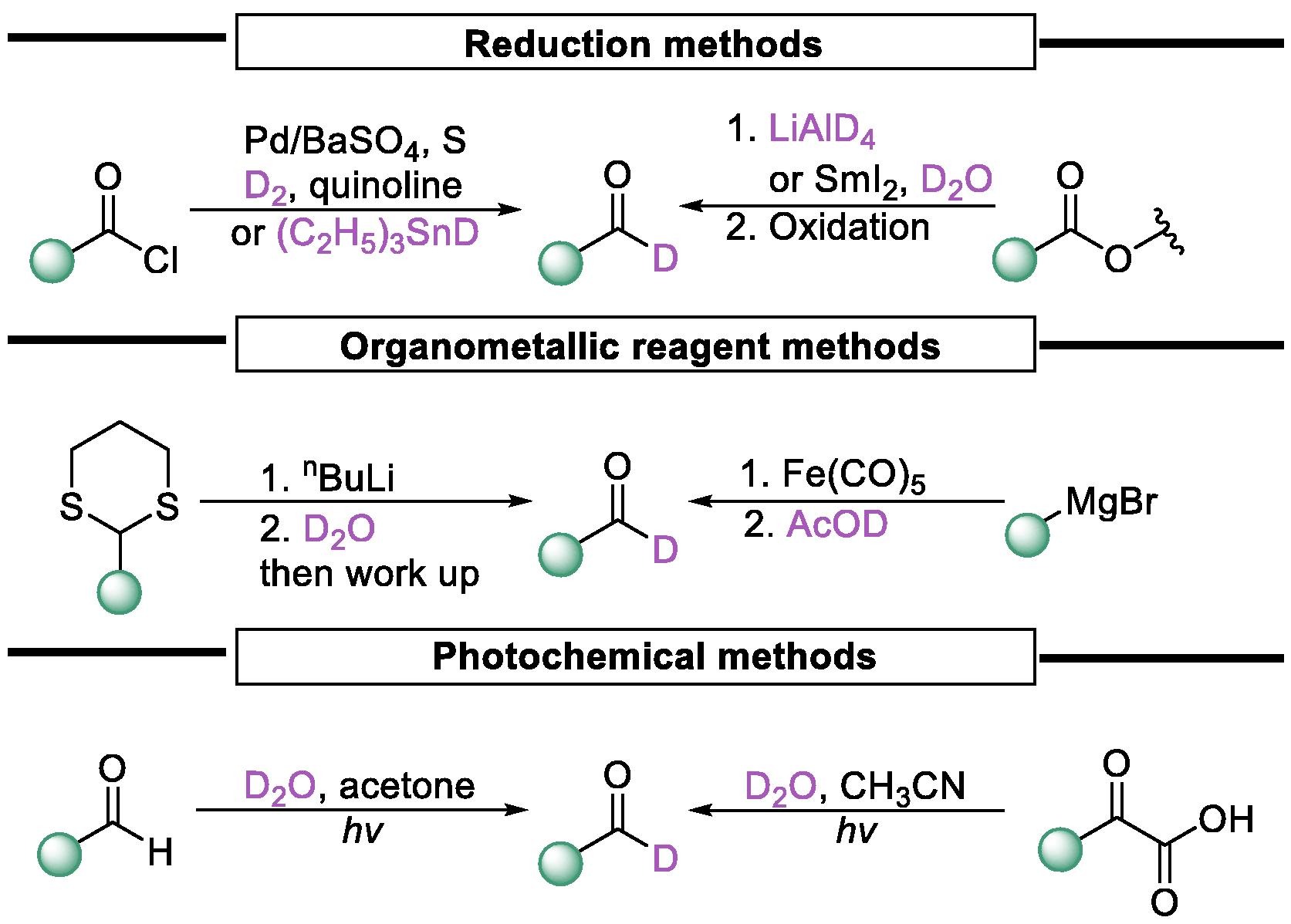 Fig.1 Classical strategy in synthesizing deuterated aldehydes.[1]
Fig.1 Classical strategy in synthesizing deuterated aldehydes.[1]
Transition-metal catalysis based methods
Reductive carbonylation reactions catalyzed by transition metals are a major strategy for the construction of formyl-CD bonds. For example, deuterated aldehydes were synthesized from aryl halides (aryl bromides and iodides) using potassium deuteride as the deuterated shuttle.
Photocatalysis based methods
Both transition metal-based photocatalysts and organic dyes can facilitate direct deuteration, offering great synthetic flexibility for potential applications.
Organocatalysis based methods
In addition to transition metal catalysis and photocatalysis, N-heterocyclic carbene (NHC)-based catalytic methods have been widely used to address the challenging synthetic task of deuterated aldehydes.
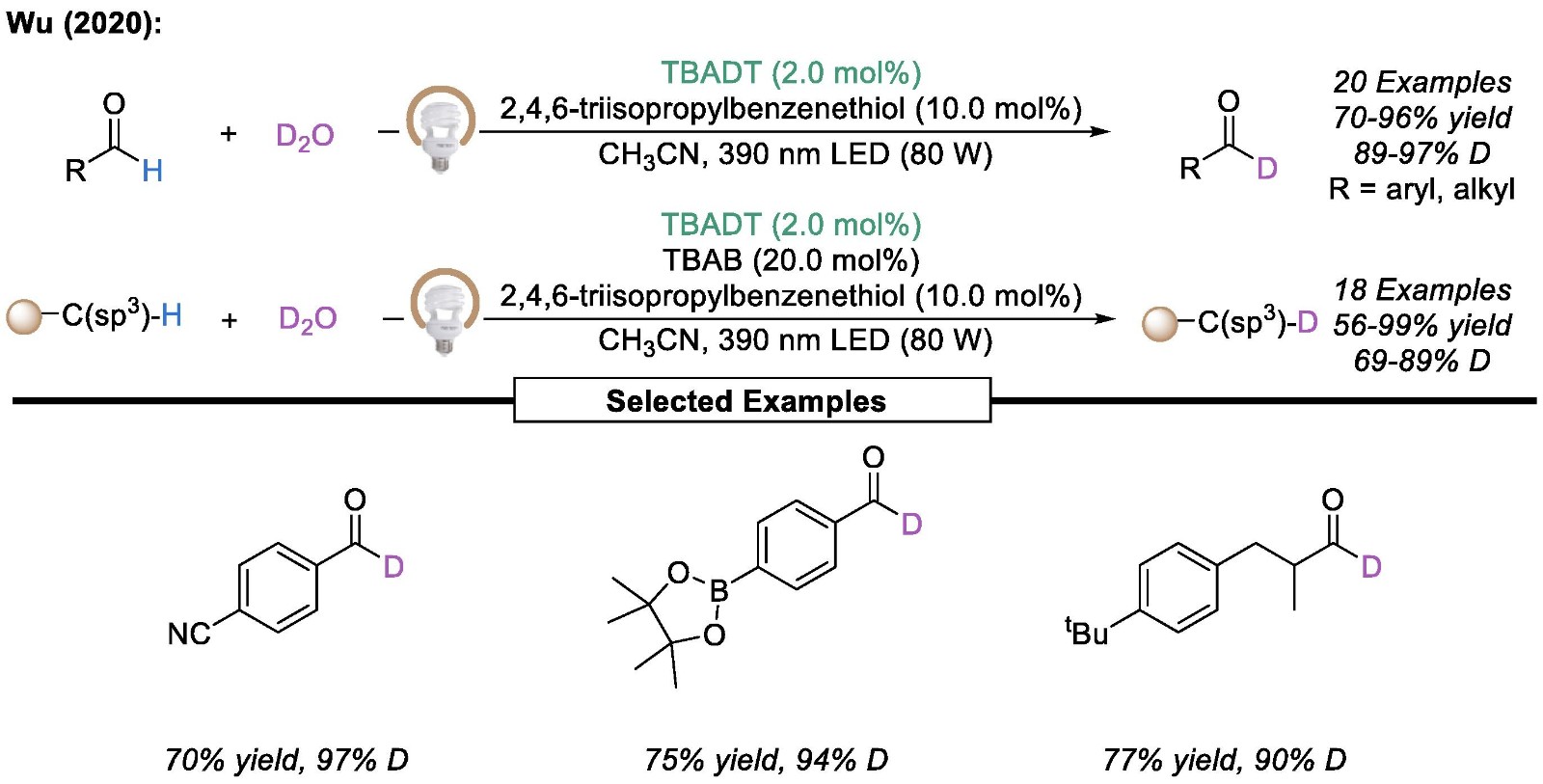 Fig.2 TBADT-catalyzed deuteration of aldehydes.[1]
Fig.2 TBADT-catalyzed deuteration of aldehydes.[1]
Miscellaneous stoichiometric methods
In addition to the above synthetic strategies, arylmethyl and cinnamyl halides can be converted to deuterated aldehydes by a one-pot method. This technique can also be used for scale-up synthesis of aldehyde-deuterated drug intermediates.
DCL™ in Deuteration of Aldehydes
Our DCL™ technology platform enables a broad substrate scope involving a wide range of aromatic and aliphatic aldehyde compounds. Specifically, benzaldehyde-based substrates with various substituents on the benzene ring, such as neutral groups (isopropyl, tert-butyl, and phenyl substituents), halogen substituents (fluorine, chlorine, bromine, etc.) , iodine), electron withdrawing group (ester group, cyano group), electron donating group (methoxyl, ether and amine substitution), trifluoromethyl and trifluoromethoxy, etc. Some sensitive functional groups such as boronic acid, alkynyl, and alkenyl are also compatible. At the same time, considering the widespread presence of heteroaromatic compounds in drug molecules, our platform is also applicable to aldehydes containing heteroaromatic rings such as pyridine, indole, quinoline, and thiazole, and can obtain products with higher deuteration rates. In addition to aromatic aldehydes, this platform is also suitable for the deuterated reaction of aliphatic aldehydes, and can obtain deuterated products with higher yields and deuteration rates.

In conclusion, our DCL™ technology platform can obtain deuterated organic compounds of various aldehydes, which is especially suitable for late deuterium modification of drug molecules, and provides an efficient and practical method for the development of deuterated drugs.
Platform Advantages
- Mild reaction conditions
- High deuterium rate
- Broad substrate scope
- Good functional group compatibility
References
- Guo Y, et al. Advances in C1-deuterated aldehyde synthesis. Coordination Chemistry Reviews. 2022, 463: 214525.
- Dong J, et al. Formyl-selective deuteration of aldehydes with D 2 O via synergistic organic and photoredox catalysis. Chemical science. 2020, 11(4): 1026-1031.
Customer Intellectual Property Protection
BOC Sciences has always regarded intellectual property as the most valuable asset of the company and its customers. We have signed non-disclosure agreements with customers and employees before the project starts, and provide synthetic route design and synthesis services in strict accordance with the terms of the non-disclosure agreement, striving to provide customers with target compounds in the shortest time possible.

 Fig.1 Classical strategy in synthesizing deuterated aldehydes.[1]
Fig.1 Classical strategy in synthesizing deuterated aldehydes.[1] Fig.2 TBADT-catalyzed deuteration of aldehydes.[1]
Fig.2 TBADT-catalyzed deuteration of aldehydes.[1]


