Deuteration of Amines and Amides Based on DCL™
Background & Overview

The demand for deuterated compounds is increasing, especially as there is a renewed interest in the therapeutic prospects of so-called "heavy drugs". This has greatly contributed to the need for new methods of H/D exchange, which are expected to have an important impact on drug discovery.
Among all deuterated functional groups, amines occupy the central position and are a group of compounds of great research interest in H/D exchange. Because these components are present in many clinically important drugs, such as acetaminophen (paracetamol), diclofenac, and acebutolol. Taking primary amines as an example, as important functional groups found in many drugs, primary amine units are usually metabolized by oxidative deamination by amine oxidase. Incorporation of deuterium at the C-H bond adjacent to the primary amine nitrogen atom can significantly improve in vivo efficacy. Incorporation of deuterium at the α-C-H position of the biologically active compounds tryptamine, amphetamine, and dopamine has been shown to be an effective way to slow metabolic oxidation.
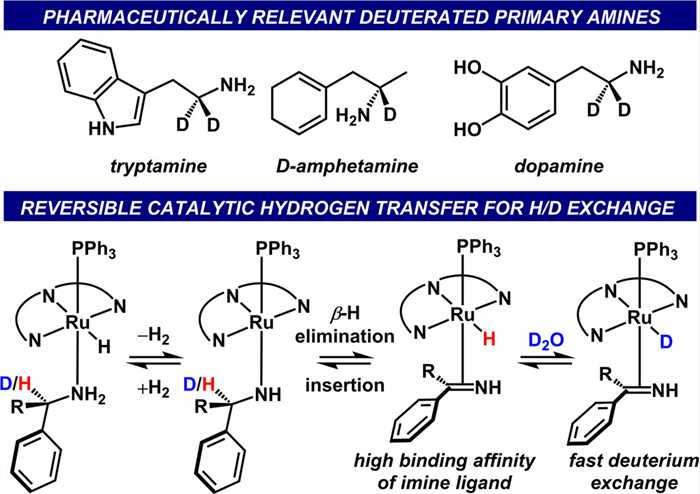 Fig.1 Select deuterated bioactive primary amines (top). Conceptual development of stereoretentive H/D exchange using hydrogen transfer (bottom).[3]
Fig.1 Select deuterated bioactive primary amines (top). Conceptual development of stereoretentive H/D exchange using hydrogen transfer (bottom).[3]
Existing Strategy for the Synthesis of Deuterated Peptides
The earliest developed deuterium-to-nitrogen associations relied primarily on the alkylation of amines or surrogates with deuterated alkyl halides or synthetic equivalents, and the reduction of nitriles, imines, and amides with LiAlD4. These classical approaches encounter limitations in efficiency, versatility, and functional group tolerance, stimulating the development of alternative processes. Other methods subsequently developed are mainly based on the direct deuteration of amines α and/or β to nitrogen atoms using metal catalysts or synergistic acid/base catalysis systems. While these approaches are attractive, they suffer from important limitations in substrate range, regioselectivity and, more importantly, deuterium incorporation. Therefore, there is a need to develop a widely applicable and versatile method for the synthesis of deuterated amines.
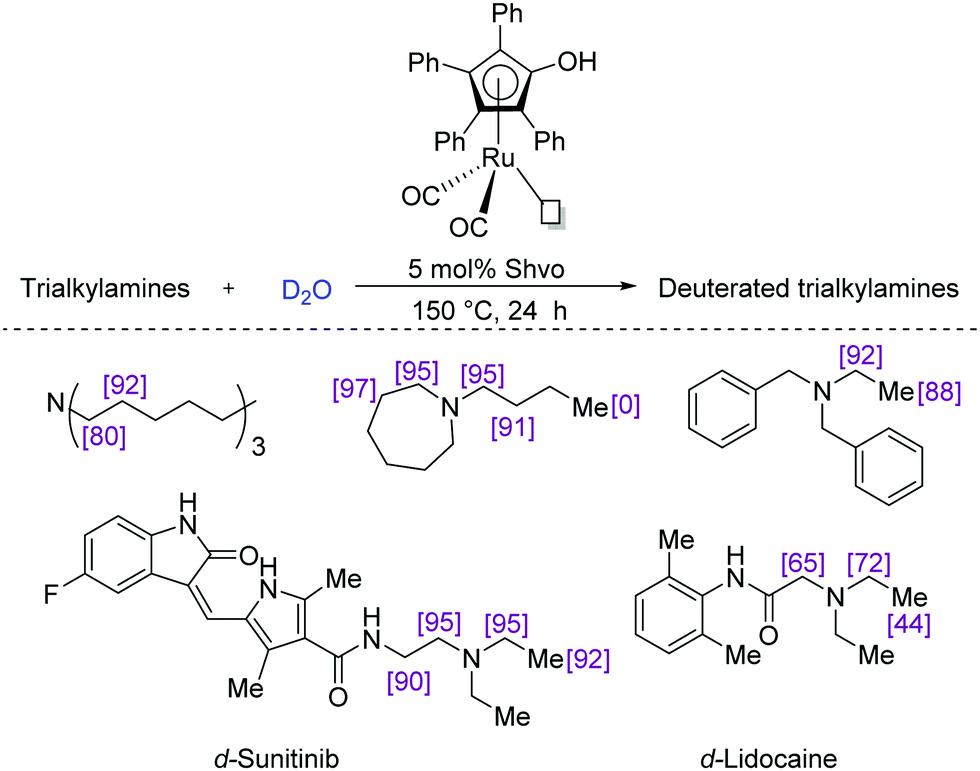 Fig.2 Ru based Shvo catalysed deuteration of amines.[4]
Fig.2 Ru based Shvo catalysed deuteration of amines.[4]
DCL™ in Deuteration of Amines & Amides
Based on the importance and growing importance of selective deuterated amines, especially in medicinal chemistry, we have developed a simple, user-friendly, and versatile method for amine synthesis that enables high-level deuterium incorporation to successfully obtain a range of amines by using an achiral hydrogen transfer catalyst. To further highlight the synthetic potential of our diverse and selective synthesis of deuterated amines, our DCL™ technology platform explored the synthesis of deuterated biorelevant amines, again in good overall yields and excellent deuterated levels. Not only that, our method can also be extended to the synthesis of selective deuterated alcohols and ethers. This illustrates that the method provided by our platform for the synthesis of deuterated amines is useful and versatile.
References
- Giles R, et al. Hydrogen–deuterium exchange of aromatic amines and amides using deuterated trifluoroacetic acid. Tetrahedron letters. 2015, 56(5): 747-749.
- Lecomte M, et al. A general, versatile and divergent synthesis of selectively deuterated amines. Chemical science. 2021, 12(33): 11157-11165.
- Hale L V A, Szymczak N K. Stereoretentive deuteration of α-chiral amines with D2O. Journal of the American Chemical Society. 2016, 138(41): 13489-13492.
- Prakash G, et al. C-H deuteration of organic compounds and potential drug candidates. Chemical Society Reviews. 2022.
Customer Intellectual Property Protection
BOC Sciences has always regarded intellectual property as the most valuable asset of the company and its customers. We have signed non-disclosure agreements with customers and employees before the project starts, and provide synthetic route design and synthesis services in strict accordance with the terms of the non-disclosure agreement, striving to provide customers with target compounds in the shortest time possible.


 Fig.1 Select deuterated bioactive primary amines (top). Conceptual development of stereoretentive H/D exchange using hydrogen transfer (bottom).[3]
Fig.1 Select deuterated bioactive primary amines (top). Conceptual development of stereoretentive H/D exchange using hydrogen transfer (bottom).[3] Fig.2 Ru based Shvo catalysed deuteration of amines.[4]
Fig.2 Ru based Shvo catalysed deuteration of amines.[4]

