Asymmetric Deuteration Based on DCL™
Background & Overview
Deuterium has long been used to alter molecular properties in a wide range of disciplines, from physics and life sciences to forensics and environmental monitoring. More recently, the potential therapeutic benefits of deuterium have been exploited to alter the pharmacokinetic properties of drug candidates and their associated metabolic profiles. Interest in deuterium drugs has increased the need to enable precise deuteration, the installation of deuterium atoms in controlled molecular locations. In particular, the incorporation of deuterium (2H) atoms at the chiral centers of compounds is becoming an increasingly desired target for pharmaceutical and analytical chemists.
BOC Sciences has extensive experience in deuterium, especially in the field of asymmetric synthesis. Our DCL™ technology platform greatly expands the toolbox available to organic chemists, providing near-perfect selectivity and mild operating conditions for the synthesis of deuterated drugs.
Our Capabilities
Due to the increasing importance of NADH-dependent reductases in chiral fine chemical synthesis, advanced enzyme evolution techniques have greatly expanded the scope of biocatalysis. However, the deuterated reducing agents currently used, such as deuterated ethanol, deuterated isopropanol, deuterated glucose or deuterated formate, are not only expensive to prepare, but also difficult to recover, which increases the cost and complexity of the process, severely limited their application in deuterated chemistry.
![Fig.1 Biocatalytic reductive deuteration requires a supply of [4-2H]-NADH.](/upload/image/2-1-14-Asymmetric-Deuteration-Based-on-DCL-1.jpg) Fig.1 Biocatalytic reductive deuteration requires a supply of [4-2H]-NADH.[1]
Fig.1 Biocatalytic reductive deuteration requires a supply of [4-2H]-NADH.[1]
BOC Sciences has developed a method for biocatalytic deuteration reactions that installs deuterium while generating chiral centers with high chemical, stereo, and isotopic selectivity. The method cites the inherent safety, cost-effectiveness, availability, and ease of handling of 2H2O as a solvent and isotopic source, combined with an appropriate reductase to reduce a series of C=O, C=N, and C=C bonds. All under mild reaction conditions and 2H can be incorporated directly from the heavy water reaction medium.
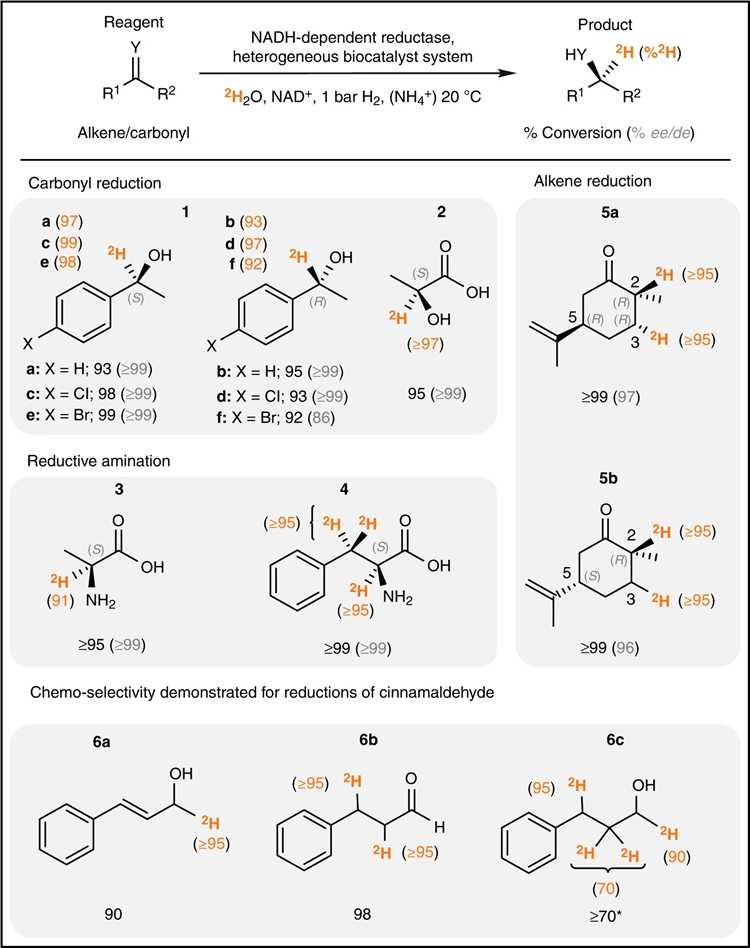 Fig.2 Scope of asymmetric biocatalytic reductive deuteration.[2]
Fig.2 Scope of asymmetric biocatalytic reductive deuteration.[2]
In conclusion, our asymmetric deuterated technology can be used in many drug molecules, such as tasalidine, cevimeline and solifenacin. We can also customize services according to customer needs.
Reference
- Rowbotham J S, et al. Bringing biocatalytic deuteration into the toolbox of asymmetric isotopic labelling techniques. Nature communications. 2020, 11(1): 1-7.
Customer Intellectual Property Protection
BOC Sciences has always regarded intellectual property as the most valuable asset of the company and its customers. We have signed non-disclosure agreements with customers and employees before the project starts, and provide synthetic route design and synthesis services in strict accordance with the terms of the non-disclosure agreement, striving to provide customers with target compounds in the shortest time possible.

![Fig.1 Biocatalytic reductive deuteration requires a supply of [4-2H]-NADH.](/upload/image/2-1-14-Asymmetric-Deuteration-Based-on-DCL-1.jpg) Fig.1 Biocatalytic reductive deuteration requires a supply of [4-2H]-NADH.[1]
Fig.1 Biocatalytic reductive deuteration requires a supply of [4-2H]-NADH.[1] Fig.2 Scope of asymmetric biocatalytic reductive deuteration.[2]
Fig.2 Scope of asymmetric biocatalytic reductive deuteration.[2]

