DCL™ for Reaction Mechanism Studies
Background & Overview

Deuterium-labeled compounds are increasingly used as research tools in life. They occupy an irreplaceable position in the development of life science, spectroscopy research and reaction mechanism research. In analytical science, deuterium-labeled compounds are widely used to study reaction mechanisms, including the measurement of kinetic isotope effects and the tracking of reaction paths. For example, the α-deuterium kinetic isotope effect was used to probe the conversion of α-mannopyranosyl triflate intermediates in the conversion of 4,6-O-benzylidene acetal to glycosides.
BOC Sciences has a general guide for the preparation of deuterium-labeled compounds, and can easily prepare useful deuterium-labeled compounds and combine various analytical equipment to study chemical reaction mechanisms and enzyme catalysis mechanisms.
What Can We Do?
Based on the kinetics and equilibrium isotope effect (KIE) of deuterium, BOC Sciences has the ability to realize chemical reaction mechanism studies and enzymatic mechanism determinations. We are committed to utilizing KIE experiments to provide useful information on all steps of the reaction mechanism.
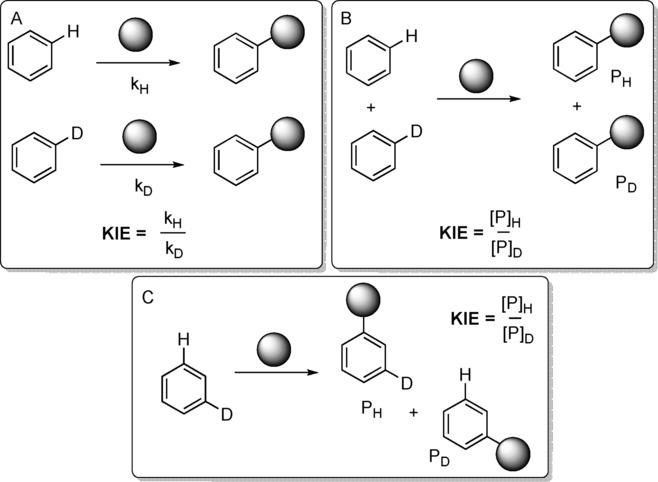 Fig.1 Complementary deuterium KIE experiments.[1]
Fig.1 Complementary deuterium KIE experiments.[1]
Since KIEs are often very sensitive to substrate and transition state structures, KIE experiments can be used to understand electronic, steric, and correlation effects that support computational hypotheses. We determine isotopic positions by NMR spectroscopy or detect mass changes by GC-MS and LC-MS, and compare the change in reaction rate after hydrogen is replaced by deuterium with the theoretical KIE value to provide essential experimental information on the calculated mechanistic pathway.
We use three different experimental designs. 1) KIEs are determined by the absolute rates of two parallel reactions; 2) KIEs are determined by intermolecular competition between deuterium-labeled and unlabeled substrates in the same reaction vial; 3) KIEs are determined by intramolecular competition, e.g. A directing group (DG) is placed between the C-H and C-D bonds.
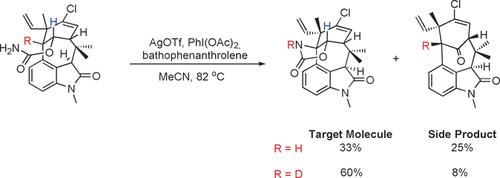 Fig.2 Strategic use of KIEs in total synthesis to facilitate C−H functionalisation.[1]
Fig.2 Strategic use of KIEs in total synthesis to facilitate C−H functionalisation.[1]
For the study of enzymatic mechanisms, we also provide powerful tools that are widely used to infer many aspects of enzymatic chemistry, such as hydrogen transfer mechanisms, oxygen activation mechanisms, and acyl transfer reactions.
Why Choose Us ?
Based on a wealth of enlightening information and unprecedented insight, BOC Sciences has gradually become an expert in deuterium technology. Since deuterium can sensitively reflect the isotopic effect of velocity theory, we use deuterium labeling technology as an important research tool to analyze the reaction mechanism.
Not only do we have the DCL™ technology platform for the fast and easy introduction of deuterium into organic molecules, but we also have a high-performance mass spectrometer for high-sensitivity detection. These will bring us closer to a true understanding of the various response mechanisms.
If you are interested in our services, please contact us immediately, then fill in the complete inquiry form, and we will reply to you as soon as possible.
Reference
- Atzrodt J, et al. Deuterium‐and tritium‐labelled compounds: applications in the life sciences. Angewandte Chemie International Edition. 2018, 57(7): 1758-1784.


 Fig.1 Complementary deuterium KIE experiments.[1]
Fig.1 Complementary deuterium KIE experiments.[1] Fig.2 Strategic use of KIEs in total synthesis to facilitate C−H functionalisation.[1]
Fig.2 Strategic use of KIEs in total synthesis to facilitate C−H functionalisation.[1]
