Deuteration of (thio)Ethers and Ketones Based on DCL™
Background & Overview

The incorporation of deuterium into organic molecules has recently received considerable attention. Deuterium-containing small molecules can be used as analytical standards and tracers to characterize new products or study reaction mechanisms. The incorporation of deuterium atoms into biologically active or drug-like molecules at specific positions may significantly affect their pharmacokinetic and metabolic properties. Over the past few decades, various methods have been developed to prepare a variety of deuterated compounds. For example, direct and stereo-retaining deuteration of primary amines using Ru catalyst and D2O as a deuterium source; dehalogenation of (hetero)aryl halides using the KOMe-disilane system, etc.
There are certain technical barriers to the synthesis of deuterated compounds because of the small library of deuterated molecules available, high cost, low selectivity in many known deuterated reactions, and not in line with modern green chemistry trends. Therefore, we have overcome many problems, and after years of painstaking research and development, we have established the DCL™ technology platform dedicated to the synthesis of deuterated compounds.
Existing Strategy for the Synthesis of Deuterated (thio)Ethers and Ketones
Deuterated (thio)ethers
In 2017, the F.DA approved the deuterium-containing compound deutetrabenazine as a clinical drug for the treatment of Huntington's disease and tardive dyskinesia, the synthesis of which typically relies on Williamson etherification with deuterated alkyl halides as the deuterium source. But it is worth noting that this reaction is only applicable to simple and less sterically hindered haloalkanes. The preparation of deuterated aryl ethers with bulky alkyl chains remains to be explored. Therefore, there is an urgent need for the development of simple and practical deuterated ether generation technologies in the drug development community.
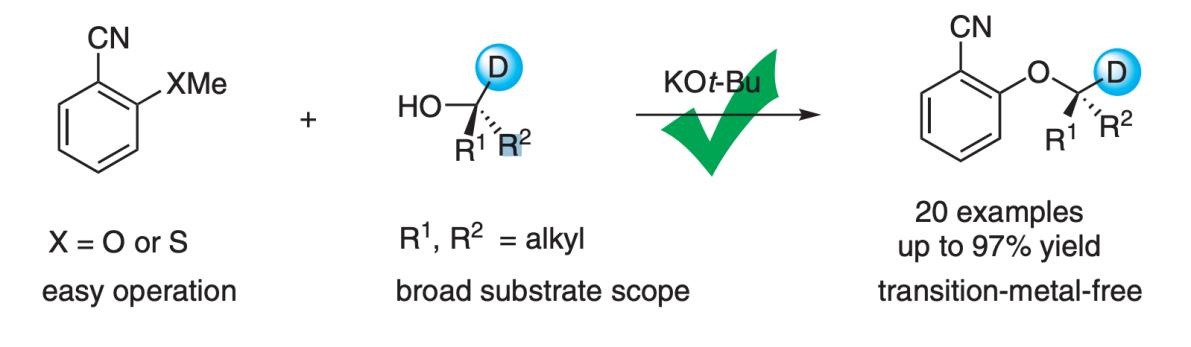 Fig.1 Deuterated aryl ether synthesis enabled by alcohol interconversion.[1]
Fig.1 Deuterated aryl ether synthesis enabled by alcohol interconversion.[1]
Deuterated ketones
Regarding the deuteration of ketones, a simple ionic liquid-driven H/D exchange reaction for selective deuteration in various relatively low-cost deuterium-containing solvents (D2O, chloroform-d, methanol-OD) combined with NMR Accurate monitoring of spectra can be an effective solution for the selective deuteration of a wide variety of compounds (alkyl and phenyl ketones).
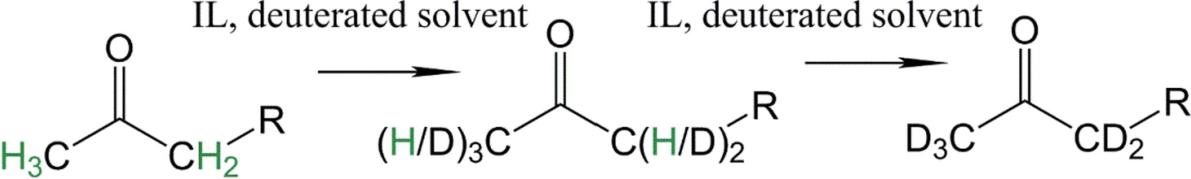 Fig.2 The reaction scheme of the model H/D exchange reaction.[3]
Fig.2 The reaction scheme of the model H/D exchange reaction.[3]
DCL™ in Deuteration of (thio)Ethers and Ketones
BOC Sciences' DCL™ technology platform provides you with the design of practical, environmentally friendly deuteration reactions to help you address your needs in drug development and industrial processes. We have successfully developed a highly efficient and transition metal-free deuteration reaction with a broad range and applicability of ether and ketone substrates. Our advantage is the easy replacement of the "H" source using mild inorganic reducing agents and an inexpensive "D" source, enabling unprecedented synthesis of deuterium-labeled molecules, achieving high levels of D incorporation, and providing only low-toxic by-products. Our technology has been successfully applied to synthesize D-labeled drug molecules such as Orphenadrine, Carbinoxamine, and Modafinil, among others, and is dedicated to opening up new opportunities in medicinal chemistry.
References
- Li S, et al. Deuterated Aryl Alkyl Ethers Synthesis via Nucleophilic Etherification of Aryl Alkyl Ethers and Thioethers with Deuterated Alcohols. Synlett. 2019, 30(15): 1805-1809.
- Cai J, et al. Transition metal-free photocatalytic reductive deuteration of ketone derivatives. Green Synthesis and Catalysis. 2022.
- Shahkhatuni A A, et al. Nmr-Monitoring Of H/D Exchange Reaction Of Ketones In Solutions Of Imidazolium Ionic Liquids. Journal of Molecular Liquids. 2022: 119746.
Customer Intellectual Property Protection
BOC Sciences has always regarded intellectual property as the most valuable asset of the company and its customers. We have signed non-disclosure agreements with customers and employees before the project starts, and provide synthetic route design and synthesis services in strict accordance with the terms of the non-disclosure agreement, striving to provide customers with target compounds in the shortest time possible.


 Fig.1 Deuterated aryl ether synthesis enabled by alcohol interconversion.[1]
Fig.1 Deuterated aryl ether synthesis enabled by alcohol interconversion.[1] Fig.2 The reaction scheme of the model H/D exchange reaction.[3]
Fig.2 The reaction scheme of the model H/D exchange reaction.[3]

